You Can Make a Solution More Concentrated by Adding
For instance if your lemonade was too tart you would add more water to decrease the concentration. A solution that can hold more solute is called.
Ml Of Solution Has A Concentration Of 3 0 M How Much Water Needs To Be Added To Dilute The Solution To 1 9 M Socratic
Then add water stir it up.

. To make a given solution more concentrated you will have to add more solute. Particles from a mixture mixing together but then eventually settling down to the bottom. Simple and easy once you think it through.
Both A and B. Design and justify a procedure for changing a solution from one concentration to another. Based on the kind of substance that has been dissolved the particles of a solute can be ions atoms or molecules.
This works through a. A homogeneous uniform mixture of two or more substances. A solution that contains the maximum amount of solute than can be dissolved in a given amount of solvent.
Increasing the solute would increase the concentration. Add universal indicator solution to a clean well in the spot plate. Because solubility depends on temperature a solution that is.
When a solution cannot dissolve more solute it is called. You can make a solution more concentrated by adding more. An atom that has a positive or negative charge.
The simplest way to change the concentration would be to change the amount of solute or solvent in the solution. The definition of dissolve is. First put gravel salt and diatomaceous earth in a cup.
Use a flat toothpick to add two scoops of citric acid to your citric acid solution to make it even more acidic. Increasing the solvent would decrease the concentration. Design a procedure for creating a solution of a given concentration.
Glass is transparent to visibile light under normal conditions. Two or more substances mixing completely so that it appears as one. The higher the temperature the more soluble most gases are in water.
The gravel should be left in the screen. 1 Show answers Another question on Physics. When a solution is saturated.
A solution has two parts a solvent and a solute. MolL can also be written in the following ways however molL or simply M is most common. A concentrated solution contains the maximum amount of solute that can be dissolved.
A solute dissolves in a solvent to form a solution. Add salt and it becomes a more concentrated solution. The relationship is as follows.
In chemistry concentrated refers to a relatively large quantity of substance present in a unit amount of a mixture. However at extremely high intensities glass will absorb most of the light incident upon it. This is a low ratio therefore the solution of MgOH2 Mg OH 2 is dilute.
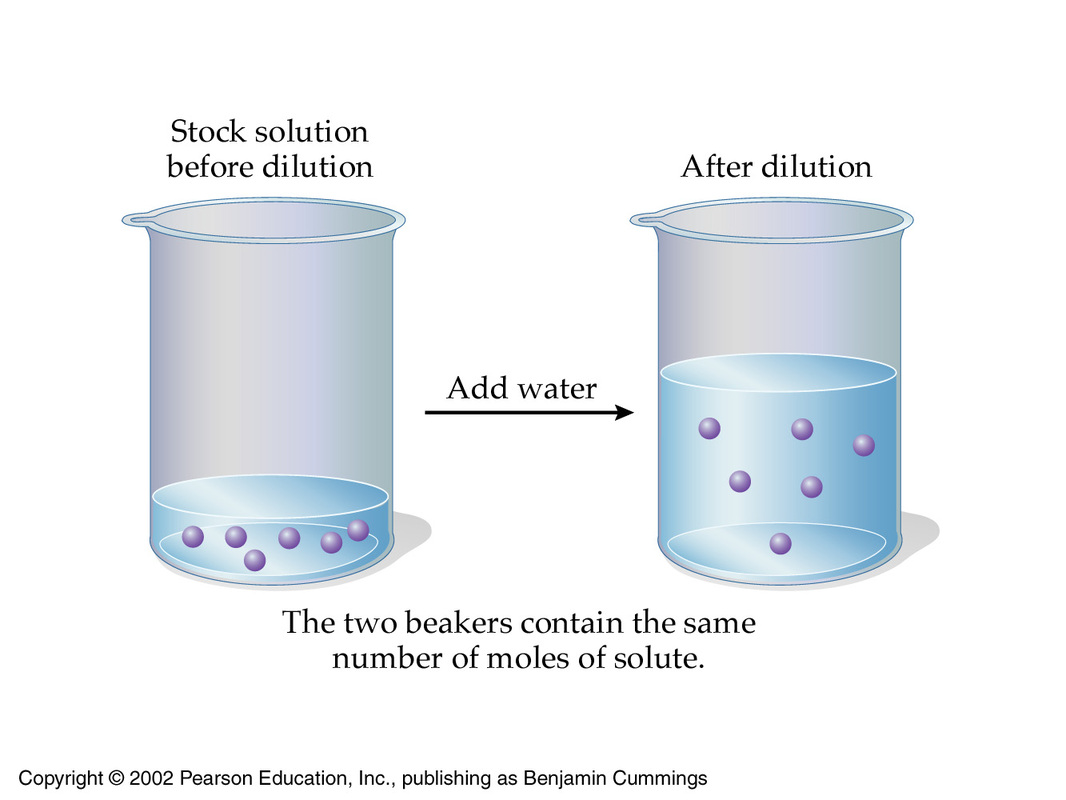
Check all that apply. A solution of concentration 1 molL is also denoted as 1 molar 1 M. The volume of solvent needed to prepare the desired concentration of a new diluted solution can be calculated mathematically.
001 001 g g of MgOH2 Mg OH 2 is added to 1 000 1 000 dm3 dm 3 of water. Explain the steps to separate a dry mixture containing gravel salt and diatomaceous earth. For example if we have a 100-milliliter cup of hot water at 100 degrees Celsius we can make a concentrated solution by adding 487 grams of sugar.
If you raise the temperature of a saturated solution you can usually add more solute and make the solution even more concentrated. The solute is the minor part of the solution. M² M¹ x V¹ V² M¹ x V¹ V¹ X whereX is the volume of water added.
Then put a screen over another cup. Add 3 drops of the more concentrated citric acid solution to the indicator and stir with a clean toothpick. A solution in which more solute can be dissolved.
On a more conceptual level this method doesnt work because it doesnt take the volumes into account. Add the two volumes together to determine the total volume of the final mixture. 100 ml 250 ml 350 ml.
Usually this means there is a lot of a solute dissolved in a given solvent. Gently swirl until the citric acid dissolves. You are given a small beaker of solution at room temp.
The initial volumes of the two solutions and the final volume of the mixed solution both affect the final concentration but this method doesnt take that into account. 100 100 dm3 dm 3 of HCl HCl is added to 10 10 dm3 dm 3 of water. This is a high ratio therefore the solution of HCl HCl is concentrated.
As you cool a saturated solution from high temperature to low temperature solids start to crystallize out of solution if you achieve a supersaturated solution. 1 molL 1 M 1 moldm 3 1 mol dm 3 1000 molm 3. If 400- ml of water is added to 100- ml of 01M NaOH then the concentration of the NaOH SOLUTION after dilution.
2 Add NaCl or other salt to shield the charges on each protein from those on. 1 Add HCl or NaOH to change the pH until these groups are mostly positively or nregatively charged causing them to no longer favor aggregation. All of these - 21296597.
This is incorrect because the answer should have been 256 M. Use the formula x c V 100 to convert the concentration c and volume V of the final solution to a percentage. If you have a solution of a chemical dissolved in water or any other solvent like ethanol for example you can make the solution more concentrated either by adding more of the chemical or by removing some of the solvent.
Next pour your mixture through the screen into the cup. Add more water and it is more dilute. Predict how solution concentration will change for any action or combination of actions that adds or removes water solute or solution and explain why.
You can make a concentrated solution more dilute by adding solvent. If V¹- ml of a solution with Molarity M¹ is diluted to a volume of V² then the concentration of the diluted Solution. Two or more substances that are not able to mix completely.
You add a bit of solute to the solution and it dissolves.

Concentrated Vs Dilute Solution What Is A Concentrated Solution Video Lesson Transcript Study Com

Solutions Solvent And Solute Teaching Chemistry Interactive Science Notebook Chemistry Classroom

Comments
Post a Comment